What is Soil pH?
Soil pH is a measurement of the acidity or alkalinity of the soil. The pH scale runs from 0 to 14. A pH of 7.0 is neutral with measurements below 7.0 reflecting acidity and those above 7.0, indicating alkalinity. It is important to recognize that the pH scale is a logarithmic one. This means that a soil with a pH of 5.5 is ten times as acidic as a soil with a pH of 6.5 while a soil with a pH of 4.5 is one hundred times as acidic as a soil with a pH of 6.5. So, while one pH unit may not seem like that much, if it is above or below a plant’s pH preference, plant growth may be limited.
Why is Soil pH Important?
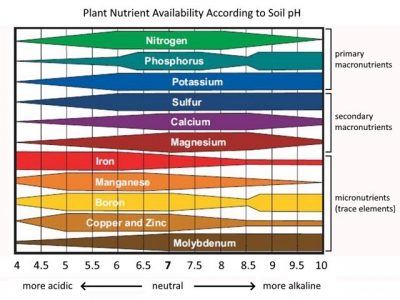
Soil pH is one of its most important soil properties because it influences nutrient availability, thereby affecting plant growth. Maintaining soil pH in the proper range is critical for success as plant growth is poor when the soil pH is outside of the desired range. An incredible number of chemical, biochemical, and biological reactions occur in our soils. Through these reactions, nutrients, whether already present in the soil or added by fertilizers, are converted into forms that can be taken up by plant roots. The pH of the soil affects these reactions and in doing so, determines the availability of the nutrients essential for plant growth. It does this by affecting a nutrient’s solubility in the water that occurs in the soil. The more soluble a nutrient is, the more that is dissolved in the soil water. Plants take up water from the soil with the dissolved nutrients in it through their roots. This is how plants get the majority of the nutrients needed for growth and development. Soil pH also affects the toxicity of certain elements and influences soil microbial activity. For example, the breakdown of organic matter is reduced in acidic soil because growth and reproduction of the soil microbes, primarily bacteria and fungi, are reduced.
Many of our native soils have a pH in the range of 4.5 to 5.5 while most of the plants we want to grow including vegetables, flowers and turf grasses are non-natives that prefer the soil pH to be between 6.0 and 6.8. Some notable exceptions are blueberries and broad-leaved evergreens like rhododendrons, which do require acid soils for good growth. Actually, it is not the acidity that these ericaceous plants need, but iron. These plants have higher iron requirements than many other types of plants and iron is soluble in the soil water when the pH is low and, therefore, more available to the plants.
When the soil pH falls below 6.0, nutrients like phosphorus, nitrogen and potassium become less available to plants. Acid soils are also typically deficient in magnesium and calcium, two important plant nutrients. Another issue with acid soils is that elements like aluminum are much more soluble and may be taken up in quantities that can harm plants. One plant that tolerates aluminum well is the blue hydrangea. In fact, aluminum is necessary for that heavenly blue color that enthralls so many gardeners.
Conversely, a soil pH that is greater than 7.5 can also render nutrients unavailable. While there are areas in Northwest Connecticut that have naturally high pH calcareous soils, this is not the case for other parts of the state. Often a very high soil pH in vegetable or flower beds indicates excessive additions of limestone, wood ashes or compost.
Managing Soil pH
Soil acidification can occur due to organic matter decomposition (e.g. crop residues, manures), the use of fertilizers containing ammonium (e.g. ammonium sulfate, monoammonium phosphate, diammonium phosphate), and the leaching of base cations (e.g. magnesium (Mg), calcium (Ca)) from the topsoil in precipitation events or through irrigation, leaving acidic cations, particularly aluminum (Al), as the dominant exchangeable cation.
Limestone Application Rates and Buffer pH
How much limestone to add depends on the soil’s present pH, the target pH for the crop being grown, and the soil’s buffering capacity, which is best described as a soil’s ability to resist or buffer itself against changes. Buffer pH measurements are made for each sample analyzed at the UConn lab enabling site specific limestone recommendations to be made. The buffer pH is a measure of the residual or reserved acidity of the soil and is dependent upon the amount of clay and organic matter in the soil. The more reserve acidity, the lower the buffer index, the greater a soil’s ability to resist change so a greater amount of limestone would need to be applied to raise the pH, (or conversely, sulfur to lower the pH).
Very acidic soils may require several applications to bring the pH up to a suitable level. As a guideline, for every 100 square feet of garden area, apply no more than 5 to 7 pounds of limestone to the surface or 10 pounds tilled into a depth of 6 inches at one time. Applications may be made at monthly or seasonal intervals until the whole recommended amount is applied. Once a desired pH is obtained, 5 pounds of limestone per 100 square feet every other year usually will maintain that level.
Regular additions of compost may eliminate the need for ground limestone. This is because the pH of a finished compost made from a variety of feed stocks is close to neutral. Manure-based composts may also contain relatively high amounts of nutrients, so it is a good idea to test the soil occasionally to make sure both the soil pH and nutrient levels are in the optimum range for plant growth.
To Raise Soil pH: Limestone is generally the material of choice to raise a soil’s pH. It neutralizes soil acidity while also adding necessary calcium and often magnesium. Dolomitic limestone, which contains both of these elements, is most widely available in Connecticut and most often recommended. If the magnesium level of your soil is above optimum, a calcitic limestone, which is composed mostly of calcium compounds, would be suggested.


Limestone can be purchased in several forms with ground, pelletized and hydrated being the most common. Economically, ground limestone is least expensive, but some do not like the dusty mess encountered when applying it. Pelletized limestone consists of pulverized limestone that is formed into little pellets. Both take about the same amount of time to react in the soil and change the pH, anywhere from 6 to 12 months or more, depending on soil and environmental conditions. Keep in mind that limestone moves very slowly down through the soil (1/2 to 1 inch/year) so it may take several years for the pH of an established lawn soil to increase. If finely ground limestone is evenly distributed in the top 6 inches of soil before planting, a change in pH will occur more quickly.
Hydrated lime is fast-acting but quite caustic and is not recommended for use by non-commercial entities. Wood ashes can also be used as a liming agent at approximately 1 ½ times the rate of the recommended limestone application. Do not leave wood ashes on plant leaves as they may be injured. Lightly water wood ashes into the soil or incorporate before planting.
There are several products available at garden centers and big box stores that claim to be fast acting and change the pH in short order. Three university studies did not find these products to elicit a pH change significantly faster than ground or pelletized limestone, however, and package directions suggest that these materials would need to be applied at more frequent intervals than ground or pelletized limestone to maintain the soil pH level. Use according to directions on label.
To Lower Soil pH: Occasionally, it is necessary to lower a soil’s pH to accommodate the growing requirements of specific plants. Typically, this is necessary when plants that evolved to tolerate low pH soils, such as rhododendrons, azaleas, blueberries, and oaks as well as many northeastern native plants are planted in soils with a pH greater than 5.0. Sulfur or aluminum sulfate are two of the most common materials used to lower soil pH. Approximately 1 to 2 pounds of sulfur per 100 square feet would be required to lower the soil pH one unit (from 6.0 to 5.0) with the greater amount needed on highly buffered soils, which contain greater amounts of clay and organic matter. Unless the sulfur can be tilled into the top 6 inches, apply at ½ to 1-pound increments (per 100 sq. ft.) at least a month apart. Aluminum sulfate would be applied at 6 times the rate of sulfur.
Soil testing takes the guesswork out of how much limestone and/or fertilizer to apply. Late summer through fall is a great time to test the soil because any limestone or sulfur recommended and applied will have time to start affecting the soil pH before the next planting season. However, soils can be tested and limestone/sulfur applied any time the ground is not frozen. If the soil pH is too high or low, it is better to add sulfur or limestone to it as soon as possible so that soil reactions can occur, and the pH can begin to change. For information on soil testing, check out www.soiltest.cahnr.uconn.edu or call the UConn Soil Nutrient Analysis Lab at (860) 486-4274.
By Dawn Pettinelli, Associate Extension Educator, PSLA & Shuresh Ghimire, Ph.D. Extension Vegetable Specialist. Updated 2021.
Questions? Contact:
UConn Soil Nutrient Analysis Laboratory
Department of Plant Science and Landscape Architecture
Phone: 860.486.4274
Email: soiltest@uconn.edu
Website: soiltesting.cahnr.uconn.edu
UConn is an equal opportunity program provider and employer.
©UConn Extension. All rights reserved.